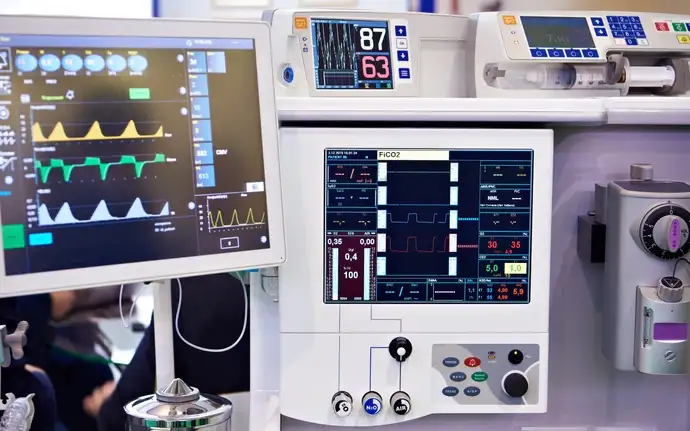
Hermetic connectors and packaging for medical devices
Innovations with an impact: Redefining medical procedures
The electronic components used in medical devices come with a complex and diverse set of requirements. These components must resist bodily fluids and withstand sterilization procedures while also offering miniaturization and advanced connections for high-frequency data. Whatever your challenge, we can work together to create innovative, fully custom designs.

Martin White
Head of Sales Office UK