
How to achieve sterility in ready-to-use pharmaceutical packaging
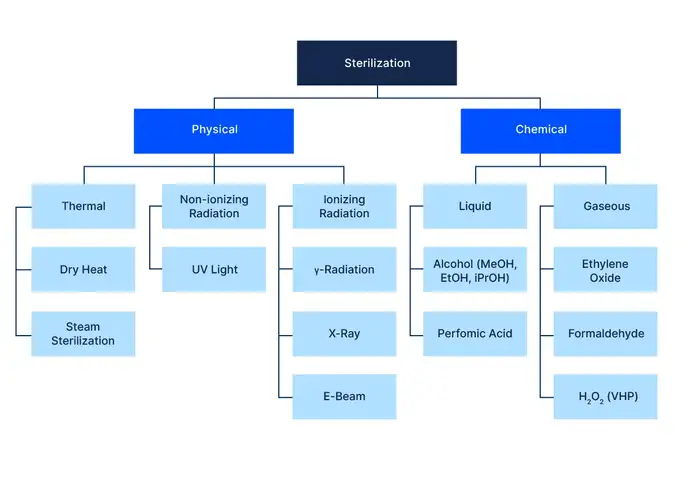
The family of sterilization techniques
From an environmental and safety perspective, steam sterilization has many benefits: the residuals are plain water, and it is relatively easy to handle compared with some of the chemical technologies. It is considered state-of-the-art for some applications, including SCHOTT's cartriQ® ready-to-use cartridges.
However, for some primary and secondary packaging containers, e.g. glass and polymer syringes, heat carries a risk for some of the components, such as plastic parts or needle glue, resulting in impairment of mechanical and dimensional stability or needle pull-out force, respectively. Therefore, the suitability of steam sterilization should be verified on a case by case basis for the goods to be sterilized, e.g. syringes.
Radiation sterilization
Ionizing and non-ionizing radiation are the other main physical means of sterilization. Non-ionizing UV light has a very short wavelength and its high energy destroys colony forming units (cfus) by cleaving chemical bonds. But although UV light sources are abundant and the technology is easy to implement, UV light offers little to no penetration and is therefore mainly used for surface decontamination, notably prior to entry into an aseptic isolator. Its low penetrability also makes it prone to shadowing, meaning that it is not effective if surfaces are not directly exposed. Hence, it requires more complex mechanics to ensure the whole surface of an object has been exposed to a high enough dose. Depending on the materials used and the dose to which they are exposed, the materials can deteriorate. But typical values used for surface decontamination are low enough for the impact on the material properties to be insignificant.
Ionizing forms of radiation are used more frequently than non-ionizing radiation. Gamma radiation offers high penetrability, is cost-effective and leaves no residuals that might create harmful side effects. However, on the downside, it turns clear borosilicate glass a brownish colour, a phenomenon called solarization, which hampers post-filling optical inspection and makes it harder to detect particles in the finished product. It can, however, be used for polymer containers, where the solarization is not as severe.
Most commonly used in the fill and finish industry is electron-beam irradiation. Its penetrability is lower than gamma radiation but higher than UV light, and it also leaves no residuals. Due to its medium penetrability, it is mainly used for low density products. As with gamma radiation, it causes solarization in borosilicate glass, and at high doses causes polymers in secondary packaging to become brittle. A big advantage of e-beam is that, unlike gamma radiation, the emission of radiation can be turned on and off as needed. This greatly improves radiation safety when handling such devices. Based on the unwanted solarization and the favourable safety profile, e-beam sources are typically used for surface decontamination with short exposure times.
Chemical sterilization
Chemical sterilization techniques are also regularly employed in the industry, since they are active at lower temperatures, i.e. 28 – 40 °C, than steam or dry heat. The lower temperature offers excellent material compatibility. However, the sterilant needs to reach every surface of the objects to achieve and guarantee an SAL of 10-6 as per ISO 14937. For gaseous sterilants, such as hydrogen peroxide (H2O2) or ethylene oxide (EtO), this means that the sterilization needs to be performed under vacuum, i.e. 1 – 10 mbar.
Vaporized hydrogen peroxide has strong oxidizing power and readily reacts with organic materials, inactivating microorganisms. As with other gaseous sterilization cycles, residuals can be an issue. This is especially true in the case of sensitive biologics, where even very low levels of residuals might degrade the product in the fill and finish process. The residual levels directly depend on the process parameters, e.g. gas concentration and aeration time.
Most widely used for the sterilization of ready-to-use (RTU) containers is ethylene oxide (EtO). While the handling of EtO requires great care, its properties make it a very potent sterilizing agent. EtO is an accepted standard sterilization technique in the pharmaceutical industry, and for many products, such as SCHOTT's syriQ® syringes and adaptiQ® RTU vials, there is no viable alternative. There are guidance and standards in place regulating EtO sterilization processes and the amount of EtO residuals allowed in a medical device: ISO 11135-1: Sterilization of Medical Devices and ISO 11993-7: EtO Residuals. All materials used in syriQ® and adaptiQ® have been selected to be compatible with EtO sterilization. The respective sterilization cycles have been designed and validated to achieve an SAL of 10-6 with minimum amounts of residuals, while at the same time reducing the amount of EtO needed, thus reducing the ecological impact.


