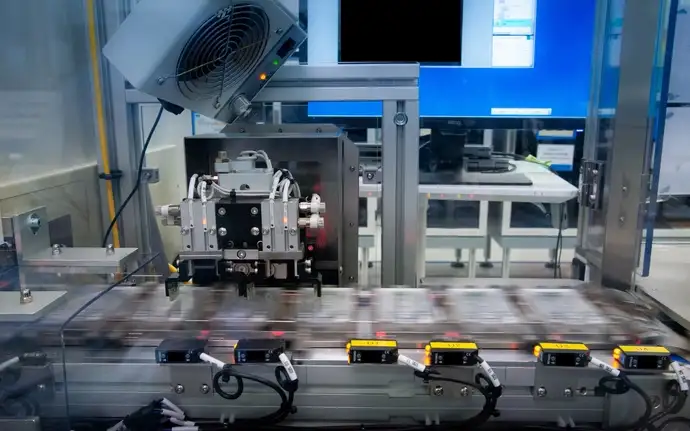
Contract product development for diagnostic and life science
You have ideas? We are here to transform them into reality. The key is to manage the product development process with precision. From concept to stable production, we have a proven process to help ensure your product’s success. We are here as your trusted advisor to ensure your products thrive in the market.
Navigating the journey from concept to commercialization
For over two decades and through thousands of successfully completed projects within life science and in-vitro diagnostics, we have honed our development process. Concept to Commercialization – a stage-gate process to accelerate your journey from early stage development to manufacturing. It is a proven path that satisfies the most challenging project needs.
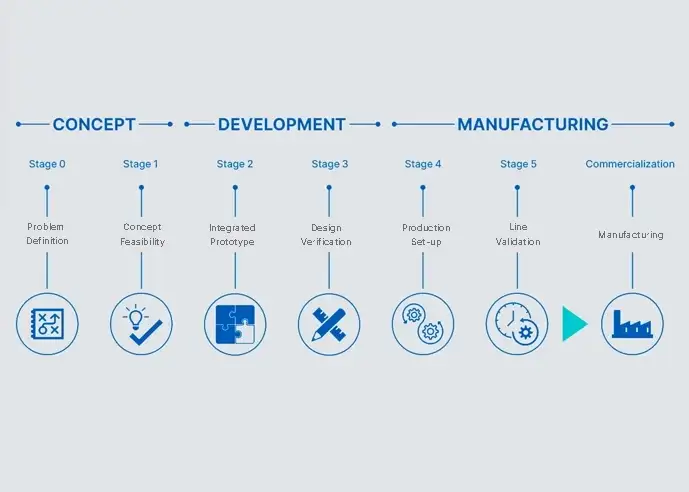
Concept to Commercialization
Services tailored to fit your requirements
We understand the product development process is not a one-size fits all solution. We are committed to meeting you wherever you are at in our development journey. We leverage our “Concept to Commercialization” process in all of our programs, but we offer different pathways based on your needs.

New product introduction pathway
Product development pathway
Research and development pathway
New product introduction pathway
Product development pathway
Research and development pathway
Utilize a cross functional team
We pride ourselves on our collaborative, multi-disciplinary approach to product development. Our team consists of scientists, mechanical engineers, application experts, manufacturing engineers, and quality engineers, ensuring a comprehensive understanding of your needs and challenges. By leveraging this diverse expertise, we not only address current requirements but also anticipate future needs, future-proofing your product against potential challenges. This proactive approach allows us to develop innovative solutions that not only meet but exceed expectations, paving the way for successful long-term manufacturing and commercialization.
Work with an established partner with global reach
FAQs
As a contract manufacturer, SCHOTT MINIFAB partners with diagnostics and life science research companies, to design, develop and manufacture diagnostic and life science research consumables based on the foundational biology and chemistry supporting the diagnostic assay. This can include Immunoassay, Molecular Diagnostics, Clinical Chemistry, Next Generation Sequencing, Nucleic Acid or Protein Arrays, Infection Disease, Cellular Analysis and more.
SCHOTT MINIFAB is your reliable contract manufacturer partner offering services ranging from product development at any stage within the product life cycle to pilot line, prototyping, or high-demand manufacturing. We offer a distinct advantage, as the manufacturer of SCHOTT NEXTERION® glass substrate, with core competencies in glass or polymer microfluidics, microarrays and assay development, for a single-source supplier from concept to commercialization. We complete the process with packaging and distribution services.
SCHOTT MINIFAB can help researchers and diagnostic companies in the development process by identifying risk and providing migration solutions, early in the development process. Our readiness assessment helps find knowledge gaps in the program and address the gaps, ensuring no downstream issues transitioning to manufacturing. This allows diagnostic and life science research companies to preserve their capital, focus on their product development, while leveraging our in-house capabilities, equipment and knowledge.
Contact us for your free consultation
Want to set up your product innovation for long-term success? Contact us for a free consultation.