Laser marking
What makes the laser marking technology special?
An indelible, permanently linked, unique data matrix code is laser-melted onto the pharmaceutical primary packaging and can be linked to unique information about the container and its contents. By using this technology, mix-up risks are reduced, recalls can be quicker and easier, and line clearance becomes much more efficient.Understand more about Laser Marking
Advantages: why Smart Containers offer efficiency and performance
Smart track and trace solutions are a major growth area in the pharmaceutical industry, and SCHOTT maintains an edge over the competition. In contrast to other solutions, the code is inextricably linked to the container at the earliest possible stage and cannot be detached without leaving a mark. This greatly increases security and ensures that the right data is linked to the right container to prevent manipulation.
How smart track and trace works
Each SCHOTT Smart Container is laser-marked with a unique identifier, enabling control of manufacturing at the most granular level possible: the individual unit. This tiny data matrix code is applied through a lasering process that ensures glass strength is retained and the code cannot be removed. It also remains stable throughout the fill-and-finish process. This method makes each container traceable all along the production line, facilitating improvements in reject management, line clearance and targeted recalls, reducing mix-ups and making lyophilization more efficient.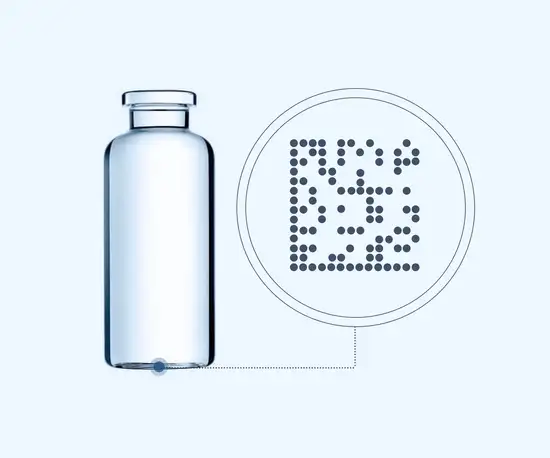
Improving pharmaceutical manufacturing, container by container
Each SCHOTT Smart Container is marked with a data matrix code that can be as small as one square millimeter, or 14 x 14 dots. Developed according to ISO/IEC 16022, the matrix can be either numeric or alphanumeric, containing 16 or 24 digits, giving a possible combination of over several sextillion different numbers.
The code is applied to the glass through a laser melting process, and it remains stable during the whole fill and finish process – through washing, autoclaving, and depyrogenation up to a temperature of 600 °C. It is also indelible and so permanently linked to the individual vial. The result is mass manufacturing of containers that remain under individual monitoring all along the production line.
YOUR ADVANTAGES
- The code is made of glass, so no new material used. This offers full traceability of the complete value chain.
- Gentle lasering process, so no increased risk of glass breakage.
- Resistant to temperature and humidity during the entire fill and finish process.
- Readability of up to 1,000 vials/min.
- Flexible and secure data management via a parallel system.
Why SCHOTT?
SCHOTT has been central to the global pharmaceutical packaging industry for many decades, developing and launching new products and technology to improve the production process and drug safety. Our smart track and trace system is just the latest in a long line of innovations that help customers increase efficiency and performance.








