
No more shattered screens
Why does glass actually break? And what do the inventors of specialty glass do to prevent glass breakage? To answer these questions, we put our glass to the test. Learn more about the exciting journey toward unbreakable glass.
The critical look upwards only hints at what's about to happen. A materials scientist presses a red button. A loud, short hiss fills the room and a clamped smartphone hurtles toward the ground from a two-meter drop. The dummy hits the hard surface without breaking and bounces several times due to the considerable height of the fall. "Oops, that was actually pretty high," the researcher comments as he picks up the test phone from the floor. Silence fills the room. With a satisfied smile, he examines the glass: "It hit hard, but it's still intact. I'm thrilled."
A visit to the glass breakers
We are in the SCHOTT test laboratory in Jena, Germany. Our materials expert's name is Jochen Alkemper. At work he is responsible for developing cover glass for SCHOTT, starting with sand and ending with strengthened glass. His job is to optimize cover glass for strength.
But what causes glass to break in the first place? And what do the glass specialists at SCHOTT do to prevent glass from breaking? Jochen explains: "Developing break resistant glass is an extremely complex process that involves a number of expert teams." At the beginning, glass is developed, followed by melting and process development. "After all, glass that looks good on paper and in the laboratory also has to be viable for mass production. Only at the very end of this process come the tests."
About Jochen Alkemper
Dr. Jochen Alkemper earned his doctorate in materials science at the Technical University of Darmstadt before joining SCHOTT in 1998. After holding several positions in Materials Development in Mainz, he took over cover glass product development in 2012, where he helped shape the company's Xensation® cover glass portfolio.

The complex art of cover glass
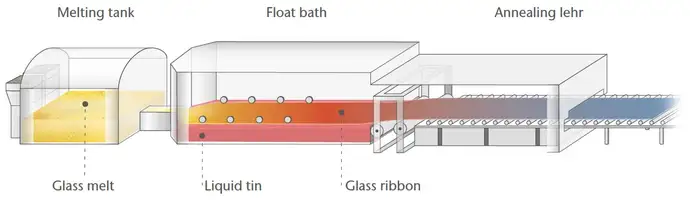
Smartphone cover glass can be manufactured using a range of production processes. "One established method is the float glass process, which we use for every shatterproof smartphone cover glass in our Xensation® product line, as well as for other technical products such as fire-resistant glass," says Jochen.
Float glass production starts with a perfectly balanced mixture of various metal oxides and sand. This mixture is melted at over 1500 °C until a viscous mass forms. This honey-like mass floats in a liquid tin bath, is drawn to the desired thickness, and then cooled in a controlled manner. This creates an extra large glass sheet, which is cut into smaller slices at the end of the process. This is referred to as raw glass.
"Glass is a brittle material, which is why it tends to break abruptly," Jochen says. He adds that its stress resistance can be significantly increased through the right post-processing. To understand this, one has to consider the atomic structure of glass. "The chemical composition of glass has just as much influence on its break or scratch resistance as the surface quality achieved in post-processing."
Modern premium cover glass is usually based on a mixture containing lithium and aluminum. That’s why this type of glass is referred to as lithium aluminosilicate (LAS) glass. A sophisticated new formulation is lithium alumino-borosilicate (LABS) glass, in which the element boron plays an important role.
Harder, better, stronger
There are several processes that can make glass more resistant. Thermal toughening, for example, is widely used in architectural glass. Here, the glass pane is homogeneously heated to a temperature just under 100 °C, which is above the transformation temperature. Then the surface is rapidly cooled, which makes the glass’s core temporarily warmer than its surface. This introduces compressive stresses into the glass, making it more robust. Alternatively, glass can be laminated, such as for car windshields. Here, at least two panes of glass are bonded together using an intermediate layer of plastic.
However, these methods are not suitable for smartphone cover glass, as it has much more extreme requirements than normal automotive or architectural applications. Cover glass has to withstand drops onto rough surfaces such as concrete. In addition, they are simply too thin for thermal toughening. The solution here is a chemical strengthening process.
Chemical strengthening – a must for robust cover glass
All cover glasses, whether it's LAS or LABS glass, need to be chemically strengthened to be robust. "We don’t temper our glasses," explains glass expert Jochen Alkemper. "Rather, we introduce compressive stresses – opposite to the tensile stresses that are responsible for breakage – into the glass through a chemical process. These make it more resistant to tensile stresses resulting from impact or bending."
One-step chemical strengthening works as follows: raw glass is immersed in a hot salt bath containing potassium ions. As glass has many sodium ions, a chemical process starts in which potassium ions replace the sodium ions on the glass surface. Since potassium ions are larger, this creates a compressive stress on the surface, which decreases in the depth of the glass as the potassium ions cannot penetrate as far. Therefore, an internal tensile stress is created in opposition to the compressive stress, i.e., an opposing force.
For the latest generation of glass containing lithium (LAS and LABS glass), a two-stage strengthening process is necessary. In the first step, the lithium ions are replaced by sodium ions. Since sodium ions are larger than lithium ions, an initial stress is introduced, before existing sodium ions are replaced by even larger potassium ions in the second step. The two-step process allows a much deeper strenghtening in the glass than a one-step process, which makes the glass particularly break-resistant, for example, against impacts.
But what happens at the molecular level when a glass breaks?
To answer this question, we visit physicist Inge Burger, an expert on cover glass strength and quality, in the SCHOTT Research & Development department in Mainz. She has been dealing with the strength of glass, including cover glass, on a daily basis for over 10 years. She tests them over and over again, but always differently.
About Inge Burger
Inge Burger holds a degree in physics. After studying in Würzburg, she specialized in testing technology and joined SCHOTT in 2007. Her specialty is the cracking of glass and its effect on strength. She has been intensively involved with the strength of Xensation® cover glass since 2010, focusing on the influence of bending, impact, scratch resistance, and cracking.

Glass surface and edges matter
"As with any glass, the surface finish is very important in preventing glass breakage," says Inge. "The more defect-free the raw glass is on the surface and at the edges, the less it tends to break." Inge is standing in front of an apparatus in which a thin, flexible glass is loaded. She pushes a button which causes the piece of glass to be bent. It cracks, breaks, and falls out of the test apparatus in small pieces.
"The test shows that this glass was not chemically strengthened," Inge explains. "That’s why it quickly capitulated under the bending stress. Let's try it again." She uses the same test setup with a chemically strengthened glass of the same thickness. Button. Motion. The glass bends – on and on. Nothing happens until the glass explodes with a bang into thousands of individual pieces.
"The pre-stress allows the glass to bend very far before it breaks," says Inge. "At that point, it is under immense tension, which discharges abruptly. That's when the ionic bonds break, the force discharges in the form of cracks, and it splinters."
This also explains the "spidering" on cover glass after a fall. If chemically strengthened glass breaks, many cracks appear simultaneously in different directions. The fracture pattern results in a spider web-like appearance. Since cover glass is bonded to the smartphone's display unit, the broken glass is held in shape.
Accidents happen
For specialty glass manufacturers, material quality is essential, as it is the foundation for all glass properties. Additionally, the raw glass quality as well as the optimization for the chemical strengthening process strongly influence glass properties. During the finishing process, it is also important to minimize defects in the glass’ surface and edges. This also applies to rugged smartphone cover glass. However, here the chemical strengthening process significantly increases the strength of the cover glass due to the introduced stresses. The two-stage strengthening process used in modern LAS and cutting-edge LABS glass is superior to the single-stage process used in other types of glass. Particularly LABS glass benefits from a dramatically improved breakage resistance, especially with regard to drop resistance, e.g. when a smartphone falls to the ground.
Why height specifications have little significance in drop tests
Modern smartphones are becoming more and more resistant to external influences like water, dust, and forces, as in being dropped. Installing glass in the device itself has just as much influence on break resistance as the cover glass type specifications, e.g. the glass bezel in the device. Device design also has a considerable influence on the glass’s performance – both positively and negatively. Also essential is the strengthened cover glass’s thickness. As a rule of thumb, the thicker it is, the more likely it is to survive drops. However, since modern smartphones are getting thinner and thinner, and glass needs to be as lightweight as possible, high-performance cover glass has to perform well even at thicknesses below 0.7 mm, which is an ongoing trade-off.

So when will unbreakable glass finally arrive?
Inge smiles: "With our latest cover glass, we are already close to unbreakable glass, but not yet at the end of what is possible. We will definitely continue to push what is physically feasible to get even closer to our vision of unbreakable glass."










