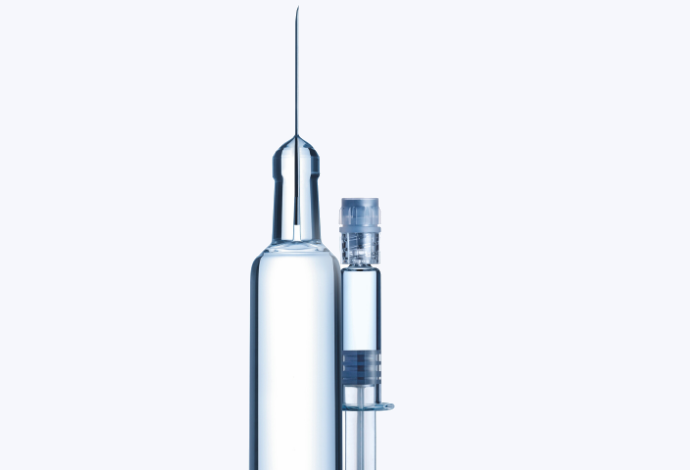
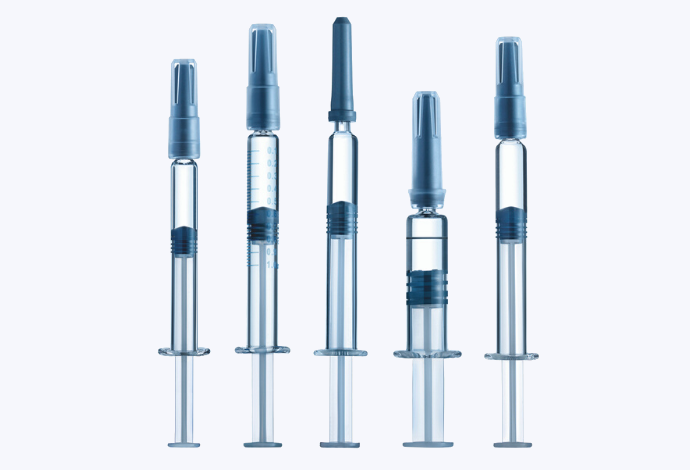
Syringes
High strength and chemical resistance
The syriQ® range is constructed from SCHOTT’s FIOLAX® glass, which features high chemical resistance, impermeability and strength. The function-improving tight dimensional tolerances of FIOLAX® in combination with low cosmetic defects results in technically outstanding syringes.
EXPLORE MATERIAL PROPERTIES IN DETAIL