Drug Delivery Systems: Vials
Dimensions and Cosmetics
With the ever-evolving significance of biotech drugs and cost sensitivity in the healthcare sector, drug delivery systems are contending with increased requirements from a market shift towards tighter regulations. Our accurate production and gentle vial handling processes facilitate tight dimensions and premium surface quality that reliably ensures your product’s shelf life.
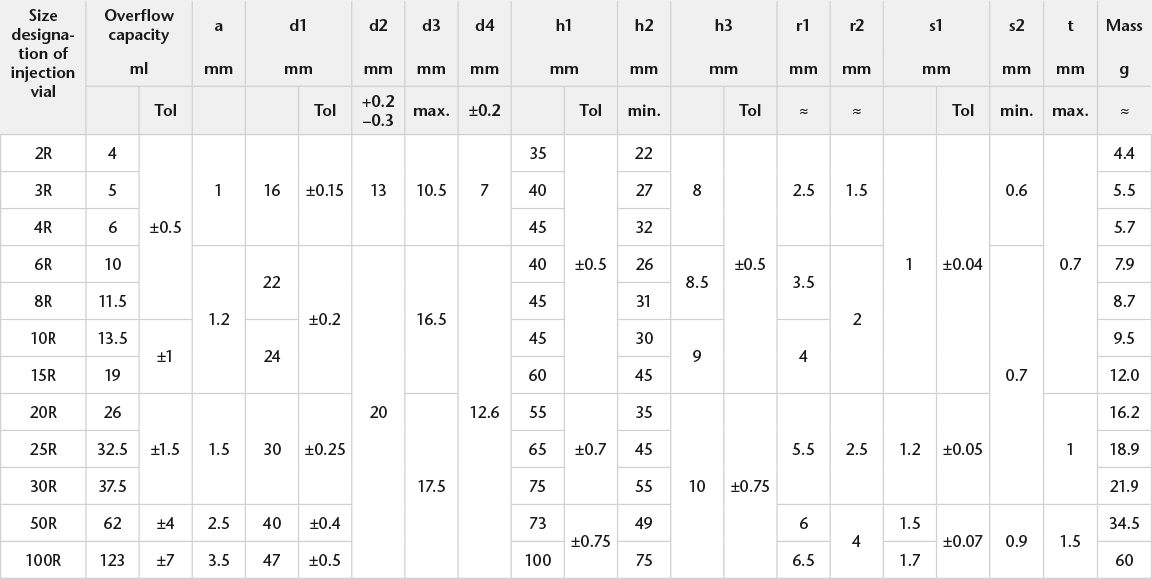
We offer tight geometric tolerances:
Hydrolytic Resistance
Improved Type I FIOLAX® borosilicate glass – FIOLAX® CHR (controlled hydrolytic resistance) – with ultralow acceptance level
Extractables and Leachables
The selection and qualification of a container for a pharmaceutical product includes extractables and leachables (E&L) testing as per international regulations.
With EVERIC® pure, we offer a solution with a unique low leachable level, especially for low-filling applications.
With SCHOTT Type I plus®, we offer an inner coating with a high barrier improvement factor against ion leaching.
Strength
EVERIC® strong uses advanced geometry to improve the production process and protects the container throughout its lifespan. Developed using advanced computer simulation techniques, these vials benefit from improved handling at key contact points, reducing the risk of glass breakage.
Material / Glass Type
SCHOTT vials are produced using a choice of glass materials, each with their own individual properties for specific applications. These materials include:
- FIOLAX®
- FIOLAX® (CHR)
- BORO-8330™
- ILLAX®
Standardized quality level according to ISO
- Production in cGMP environment
- Statistical in-process control
- Dimensional and cosmetic AQL levels according to ISO
- 100% camera inspection of dimensional parameters
- 100% camera inspection of cosmetic defects in dedicated sites
Customized product specifications and quality levels
- Customized dimensional and cosmetic AQL levels
- 100% camera inspection of dimensional and cosmetic parameters
Available product variants are:
- SCHOTT TopLyo®
- SCHOTT Type I plus®
- EVERIC® pure
- EVERIC® strong
- EVERIC® smooth
- adaptiQ®

Florence Buscke
Sr. Global Product Manager