Pharma Analytics
Chemical durability
The chemical durability of primary packaging is vital in preventing drug-container interaction. Such interaction can lead to glass delamination, particle formation or leachables from the container, resulting in market recall. SCHOTT pharma services offers a range of analytical tests to assess any potential risks and find strategies to avoid them, including drug compatibility and delamination studies, container inspection, and particle analysis
Extractables and leachables / system performance
International regulations stipulate that the selection and qualification of a pharmaceutical container includes extractables and leachables testing. Using a wide range of state-of-the-art equipment and highly sophisticated analytical methods, SCHOTT pharma services performs extractables, accelerated leachables and real-time leachables studies, as well as elemental impurity analysis and system performance testing of the packaging components.
Compendial tests
SCHOTT pharma services offers material conformity testing according to current USP, EP, and JP guidelines, along with selected ISO, ASTM, and in-house methods. The material conformity testing of pharmaceutical containers assists pharmaceutical companies in ensuring that the primary packaging container complies with pharmacopeia regulations. Samples are drawn from production lots and tested according to stipulated methods.
Specialized analytics for pharmaceutical packaging
With over 40 years of experience in analytics and specialized testing of pharmaceutical packaging, SCHOTT pharma services offers a combination of expertise and state-of-the-art analytical technology. Supporting customers worldwide, our testing conforms to all current EP, USP and JP regulatory guidelines.
Chemical durability
SCHOTT pharma services offers a range of screening packages using specialized analytics to assess the risks of drug-container interaction and establish strategies to avoid them.Container inspection and screening
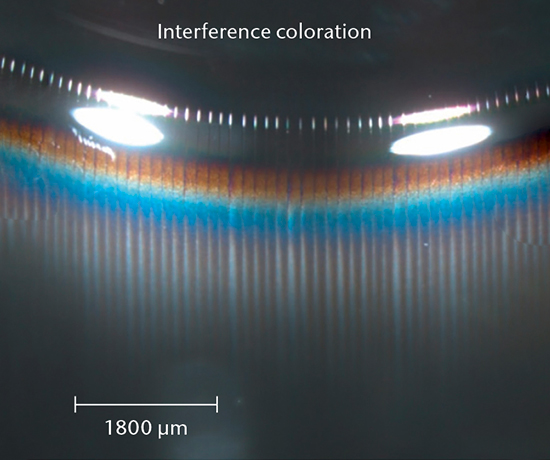
Glass delamination screening begins with visual inspection by eye and camera methods to detect flake-like particles. After emptying the container, stereomicroscopy is used to look for altered regions of a container for further surface analysis to determine the most affected samples out of a set.
Glass delamination confirmation
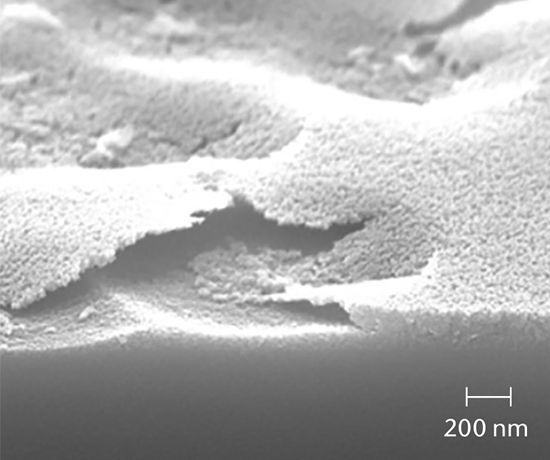
SEM cross-section analysis in combination with stereomicroscopy is used to determine the propensity for delamination (early indicators of delamination and delaminated areas) and the extent of corrosive attack of the inner glass surface.
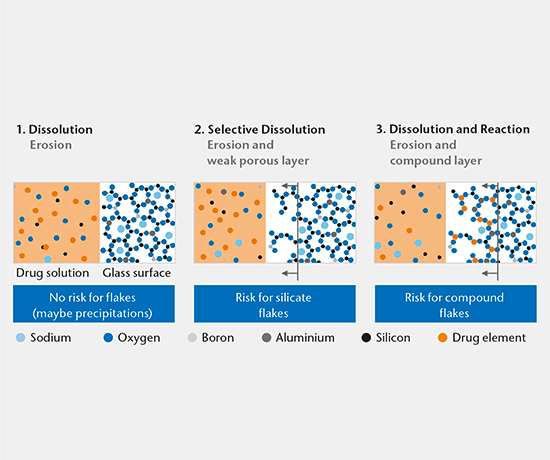
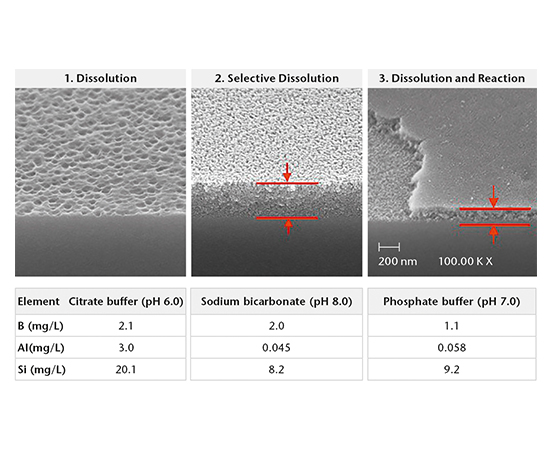
Drug-container interaction
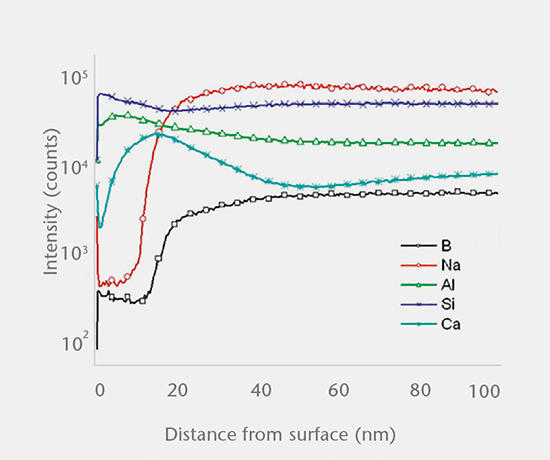
SIMS depth profiling, SEM cross-section analysis, and ICP-OES/MS solution analysis are used to determine the mechanism of drug-container interaction. There are three main mechanisms:
- Dissolution
- Selective dissolution
- Dissolution and reaction
Predictive and real-time delamination studies
SCHOTT pharma services has developed a delamination screening package in accordance with USP <1660> to assess the likelihood of delamination during the shelf life of the product. A combination of tests investigating the container surface, the near-surface region, and the solution determine the risk of glass delamination.
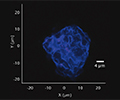
Particle analysis
Particulate analysis (inorganic)
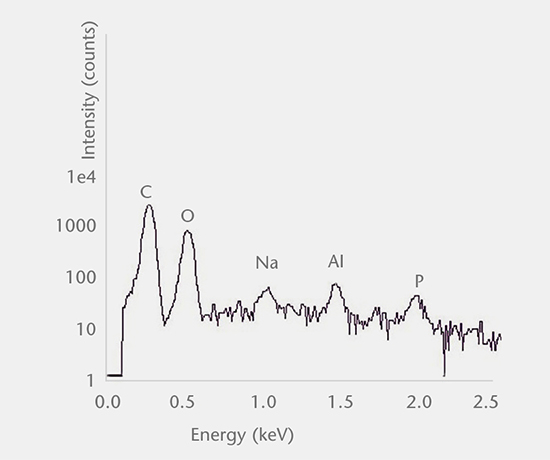
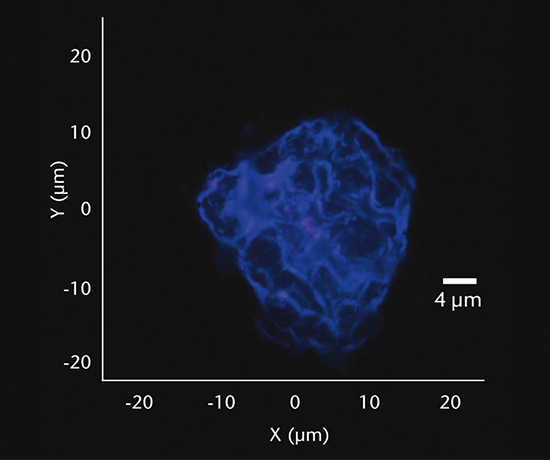
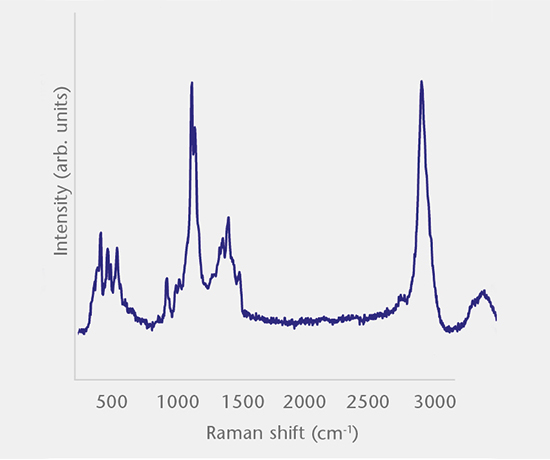
Inorganic particles need to be isolated via filtration and analyzed for chemical composition by SEM/EDS, Raman microscopy, FTIR and morphology by SEM-EDS to identify and determine the root cause. Common sources of inorganic particles include manufacturing byproducts, deposits from processing, particles from breakage, and flake-like particles from delamination.
Particulate analysis (organic)
Organic particles need to be assessed in the same manner as inorganic. Common sources of organic particles include those of human nature (skin, hair), fibers (clean room cloth, filters), and formulation precipitates or secondary packaging material, such as polymer boxes or shrink wrap.
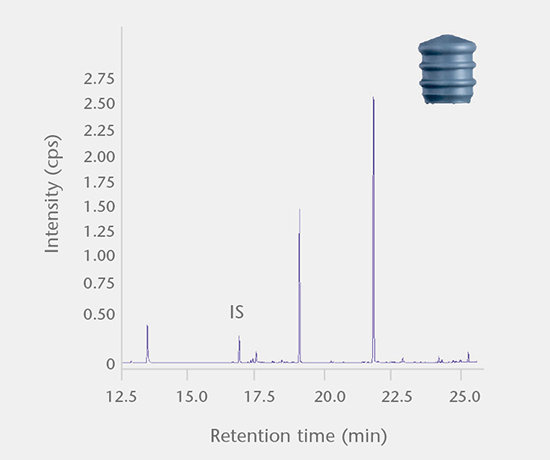
Extractables and leachables / system performance
The SCHOTT pharma services team has developed a range of E&L tests for pharmaceutical packaging that fulfill the requirements of international regulatory agencies.Extractables and leachables
Extractables studies
Extractables studies determine the amount of organic and inorganic substances extracted from primary packaging materials that could potentially migrate into the drug product. Study protocols are based on the most recent guideline recommendations of USP <1663>, USP <1664>, EP, ISO 10993, and PQRI.
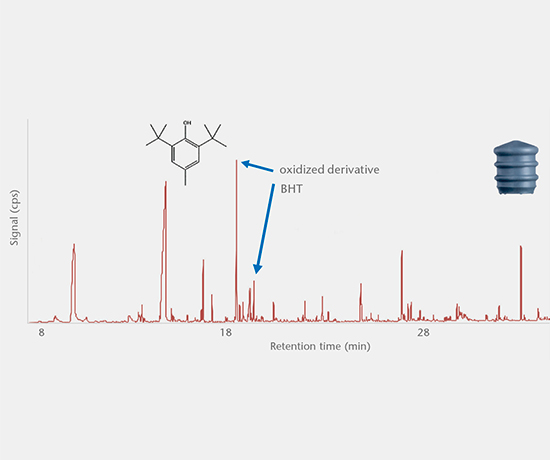
Accelerated and real-time leachables studies
These studies involve method development and validation followed by the determination of leachables and cross-reaction products in a drug after storage in a closed container under accelerated and real-time test conditions. All analyses are performed using state-of-the-art equipment. Our interpretation of the E&L studies includes guideline expertise (e.g. ICH M7) for effective assessment of the results. Further support for the toxicological assessment of E&L data is available from a collaboration partner.
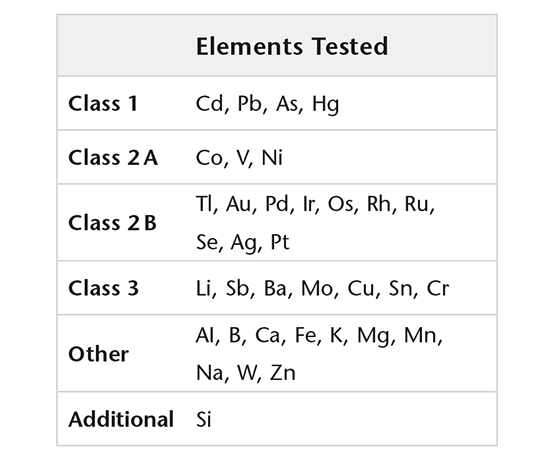
Elemental impurities
Elemental impurities (USP <232>, ICH Q3D) are determined using validated methods by high resolution ICP-MS, while extractable and leachable silicone is determined by GF-AAS.
System performance tests
System performance tests
SCHOTT pharma services’ expertise in system performance tests is based on long-term experience and a deep knowledge of glass, coatings, polymer and elastomer components.
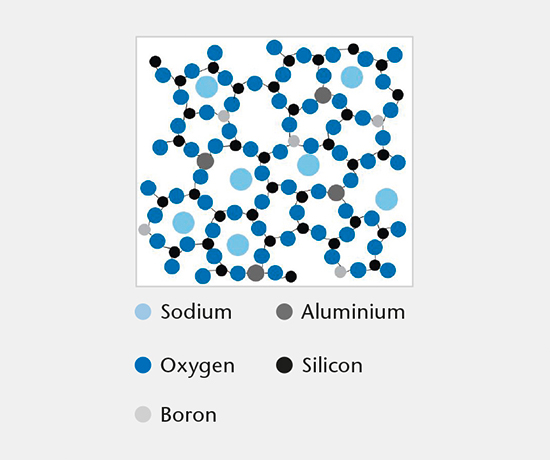
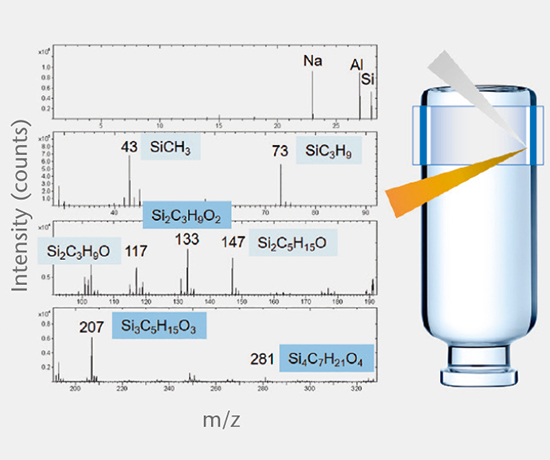
Glass composition
This test identifies the chemical glass composition of primary packaging containers and manufacturer based on a comprehensive database of published glass compositions.
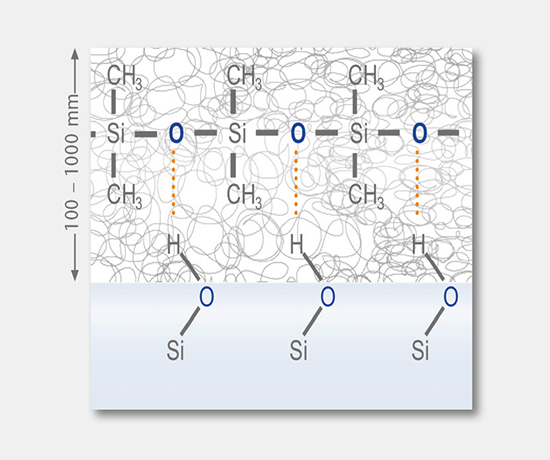
Siliconization, coating and treatment
These tests involve the determination of applied coatings and treatments, such as silicone oil, barrier coating, hydrophobic coating, and the chemical strengthening of glass containers.
Rubber characterization
Rubber materials and coatings are identified using a combination of analytical methods.
Compendial tests
SCHOTT pharma services offers a wide range of analytical tests for pharmaceutical packaging, as well as detailed knowledge of international regulations and standards. All our testing methods comply with international pharmacopeias (USP/EP/JP) and industry bodies (ISO, DIN, ASTM), and we are available to offer advice and support during pharmacopoeia testing.
Glass packaging components compliance
The material conformity tests of pharmaceutical glass containers assist pharmaceutical companies in ensuring that the primary packaging container complies with pharmacopeia regulations. Samples are drawn from production lots and tested according to stipulated methods. The range of compliance test methods available for glass containers includes USP <660>, USP <211>, EP 3.2.1 (alkalinity, hydrolytic resistance, arsenic), as well as ISO 9626 and ISO 15350 (conformity of syringe needle diameter and composition).

Elastomeric closure components compliance
The material conformity tests of elastomeric closures for pharmaceutical packaging assist pharmaceutical companies in ensuring that the elastomeric closure complies with pharmacopeia regulations. Samples are drawn from production lots and tested according to stipulated methods. The range of compliance test methods available for rubber components includes EP 3.2.9. (physicochemical tests) and ISO 8871 (particle count, UV, ash, density).
Downloads
How we proceed
SCHOTT pharma services’s team of specialists is available to offer support and advice about your pharmaceutical packaging challenge. We offer a complete process that will take you from initial request to effective solution.
Why you should work with SCHOTT to create the perfect packaging