Pharma Packaging Consulting and Development
Regulatory services
Our Regulatory Affairs (RA) Services Team offers support with the seamless integration of our pharmaceutical packaging into filing activities for new drug products in your country or expansion into new countries. As per national regulatory requirements, we can prepare Drug Master Files (DMF), national product registrations and packaging dossiers that cover all necessary information about the pharmaceutical packaging applied for your drug or medical device product. We are offering further support to clarify content, filing strategy, and the regulatory requirements related to your filing process and interaction with authorities. For this purpose we provide trainings, options for team-up between your and our RA-Team as well as creation of customized documents.
Research and development
Our product platforms rely on development activities, which fulfill the highest standards for quality and time-to-market from study to industrialization. We are a trusted partner when it comes to developing solutions tailored to your individual needs. Our R&D services support you to create and verify your container from first feasibilities to final for-human-use product.
Mechanical stability
SCHOTT pharma services is a solution provider for glass breakage issues and the mechanical stability testing of pharmaceutical containers. Our experts in fractography and strength testing can identify the root cause of glass breakage and determine the fracture probability based on our large portfolio of standard and customized in-house testing methods.
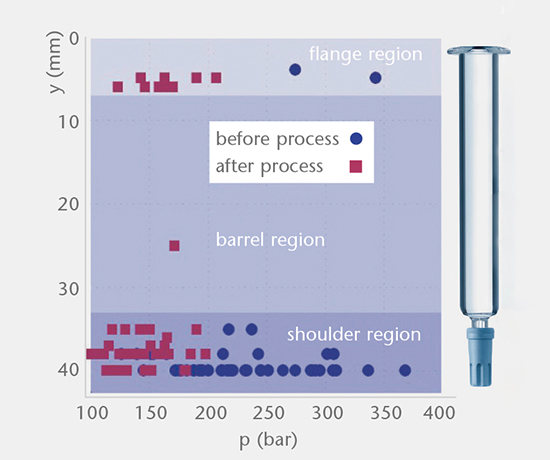
Smart Skin
Smart Skin is a state-of-the-art system for measuring line pressure on filling and packaging lines. Using drone containers and sensors on the line, the effect of pressure can be monitored in real-time and analyzed to understand its impact on performance. The strong cooperation between SCHOTT and Smart Skin enables accurate optimization of the fill + finish process to reduce glass breakage, bruising and micro fractures, and increase efficiency.
Discover how we can optimize your pharma packaging
Complete optimization of line performance is the ultimate goal of everyone involved in the pharmaceutical packaging industry, and SCHOTT offers a number of ways to increase the efficiency of your fill and finish process.
We also guide customers through the entire drug registration process by providing a range of regulatory services, and support them during the development and testing phases, all following the latest international standards.
Regulatory Services
SCHOTT Pharmaceutical Systems offers a wide range of regulatory services designed to make your drug registration process as smooth as possible.Drug Master File
In the USA, Canada and China, pharmaceutical packaging products are regulatory covered by Drug Master Files (DMF), separately from the drug or medical device product. Our Regulatory Affairs and Compliance Team takes care of the generation, filing and updating of DMFs. Our DMF filings in the USA and Canada are generated and maintained in the electronic Common Technical Document (eCTD) format, while DMF filings for China are created and maintained bilingually in the China-specific dossier format.
For integration into the filing of your drug or medical device product, we’ll provide you a Letter of Authorization (LOA). Based on this LOA, national authorities are authorized to apply the information of the referred DMF as packaging information while reviewing your drug or medical device application. This procedure offers you the flexibility of using the same LOA and DMF reference to apply for other products using the same pharmaceutical packaging.
In some cases of brand new products or expansion to new countries, new DMFs may have to be filed and reflected in your filing timeline. Our sales and product development teams initiate regulatory review in early project stage to assure new filings are available on time.
How to access
You can request your LOAs via an online request form. Based on customer entries, we’ll generate and provide a signed LOA using national LOA template documents for a fast and smooth drug approval process.
National Packaging registration
For countries that require separate product-specific registration of packaging materials, our Regulatory Affairs and Compliance Team takes care of the generation, filing and updating of the registration. In these cases, the packaging is registered separately from the drug or medical device product.
For integration into the filing of your drug or medical device product for those countries, our national product registration is available without further authorization. This procedure offers you the flexibility to use the same reference when applying for other products using the same pharmaceutical packaging.
In some cases of new products or expansion to new countries, new national packaging registrations may have to be filed and reflected in your filing timeline. Our sales and product development teams initiate regulatory review in early project stage to assure new filings are available on time.
How to Access
You can request the availability and preparation of national product registrations via our contact form below. Please provide the following information in your request:
- Your country of interest.
- Drug or Medical Device product.
- SCHOTT packaging product used for filing.
- Intended submission date.
Packaging dossiers
For countries in which the pharmaceutical packaging is part of the drug or medical device product registration and not a separate filing or registration (for example, the EU), our team provides you with a packaging dossier. These dossiers follow the standard structures of your drug or medical device filing process (ICH CTD granularity) and are available in eCTD format for initial creation, as well as maintenance. By doing this, we minimize your integration effort, as well as reducing the risk of authority requests or delays during registration approvals.
In some cases of new products or expansion to new countries, new dossiers may have to be set up. Our sales and product development teams initiate regulatory review in early project stage to assure new filings are available on time.
How to Access
You can request the availability and preparation of national product registrations via our contact form below. Please provide the following information in your request:
- Your country of interest.
- Drug or Medical Device product.
- SCHOTT packaging product used for filing.
- Intended submission date.
For all our regulatory services our experts offer trainings or team-up with your team to clarify content, regulatory requirements and best-practices as well as assist in integration strategy.
Training
We offer training through on-site or web-based consultation, as well as via our education platform PHARMAVERSITY. Trainings cover global regulations and filings for optimal integration of primary packaging into your drug or medical device application.
Trainings deliver insights into regulatory changes, deliverables, and best practice in related filing to customers. Trainings are focused on the country of interest and type of registration (drug product or Medical Device Registration). This helps customers initiate the correct steps for their registration by providing a structured overview of the procedures in this rapidly changing regulatory environment.
How to access
Please use the request button to request further information or a proposed training program, and provide the following information in your request:
- Your countries of interest.
- Intended focus: Drug or Medical Device?
- SCHOTT packaging products of interest
Team-up with our Regulatory Services team
In addition to training, we can team up with your project or regulatory affairs team to:
Address specific questions related to our products and their regulatory documentation.
Set up a suitable filing or national registration strategy.
Solve current or urgent issues.
A team-up can range from a single joint call or on-site workshop to a cooperation project over a certain period. As a leading provider of pharmaceutical packaging solutions, we offer a depth of experience from previous challenges.
How to access
Please use the request button to request further information or a proposed team-up program, and provide the following information in your request:
- Your countries of interest.
- Intended focus: Drug or Medical Device?
- SCHOTT packaging products of interest.
Customized documents
While we have set up our filing, registration, and dossier documentation to ensure all essential requirements are met, some details of your drug or medical device product may require additional information to promote your filing or inquiries by the national authorities. In this case, we’re happy to discuss your specific needs and how we can best support you.
How to access
Please use the request button to explain your requirement for documentation exceeding standard filing activity, and provide the following information in your request:
- Brief description of topic, background and intended purpose.
- Your country of interest.
- Drug or Medical Device Product.
- SCHOTT packaging product of interest.
Contact for regulatory services
Do you have any questions or requests on regulatory services? Please get in contact with us: regulatory.pharma@schott.comProduct development and functionality testing
All SCHOTT pharmaceutical packaging undergoes a strict development and testing process, following the latest international standards and quality guidelines.Product development
To guarantee that our products are safe and easy to administer for patients and healthcare personnel worldwide, our development process follows strict quality guidelines and complies with the latest international standards and regulatory requirements. SCHOTT innovation is driven by a variety of experts from various fields and the seamless integration of product development within the global organization.
Product functionality testing
Proven functionality of SCHOTT pharmaceutical packaging solutions is crucial for patient safety and a trouble-free user experience. With our broad range of validated in-house and ISO test methods, and trusted network of external testing partners, we minimize customer risk by using extensive Design Verification testing. Analytical methods follow the latest international standards.
Customer projects
For customized variants of our platforms or complete customer-specific innovation projects, you can rely on SCHOTT. From requirements engineering through the whole development process, up to the availability of products for human use, we are a trusted partner around the world – on various levels of customer integration based on individual needs.
Mechanical stability
Mechanical stability tests are the basis for weak point analysis of production lines and container design.Fractography and breakage analysis
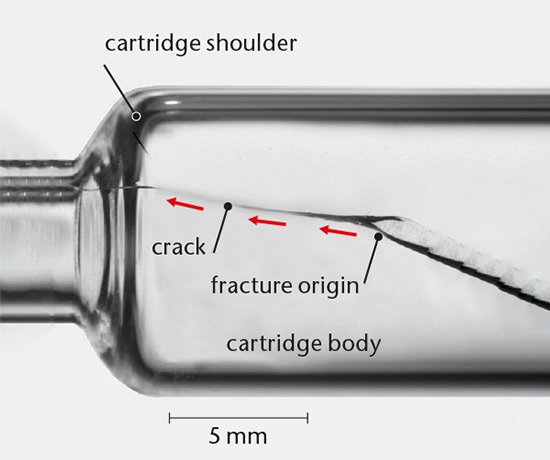
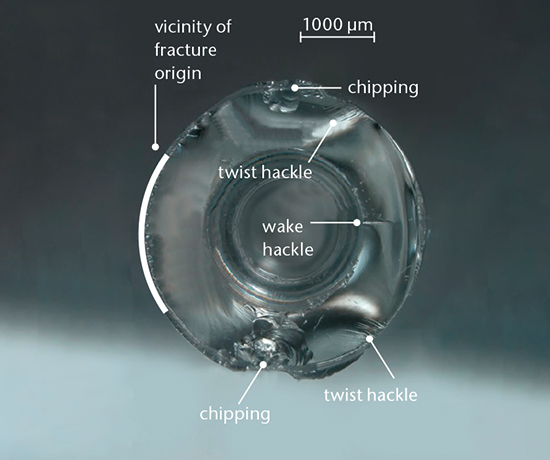
All broken glass samples and material cracks tell stories and leave behind clues. By using optical and scanning electron microscopy we can determine the origin and propagation of glass breakage. Clear evidence of the root cause can then be drawn and the applied force that led to the failure can be determined. Integrity of the glass container can be additionally assessed by dye penetration testing.
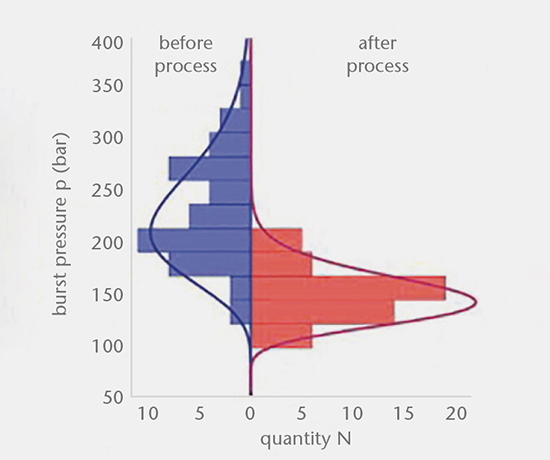
Container strength testing
The strength of glass products isn’t just a material property, but also depends on the individual quality of the surface. Strength testing enables the prediction of fracture probabilities of glass containers and forms the basis of a risk assessment for a specific container system. Samples of different manufacturers and lots can be compared and evaluated, and the influence of processing steps can be analyzed. Burst pressure testing reveals the weakest spot of a container, while specific tests target critical areas such as the flange or cone. Testing is carried out using customized testing methods alongside comprehensive statistical evaluations.

Training course for fractography and strength
It’s said that glass doesn’t forget. Any mechanical impact such as glass-to-glass contact will be saved as surface defects, and this damage will enhance the probability of breakage. Additional risks come from stress introduced during processing steps such as lyophilization or hot-forming, but advanced analytical techniques can reveal the stress points in order to determine weak spots. SCHOTT run a two-day on-site course in which you can learn all about the various mechanical stability tests for glass. This detailed, hands-on course includes training in glass production, testing, glass properties, fracture mechanics and statistics, strength testing and Weibull distributions, fracture patterns, fracture surface markings, sample preparation and imaging techniques.
Optimizing the fill + finish performance with Smart Skin
As valued partners of SCHOTT, Smart Skin offer in-depth analysis of your filling and packaging lines, providing precise pressure management to reduce glass breakage, bruising and micro fractures, and optimizing performance.
At a glance
- Reduce vial breakage, bruising and microfractures
- Improve flow and cost maintenance
- Use as an engineering tool to validate line improvement
Our Smart Skin partners
Together with our partners, we discussed how data management with Smart Skin’s drone technology helps to improve the line performance of key players in the industry. We learned from the best and gained insights for our own purposes, sharing knowledge and discussing future developments to grow the business.
At a glance