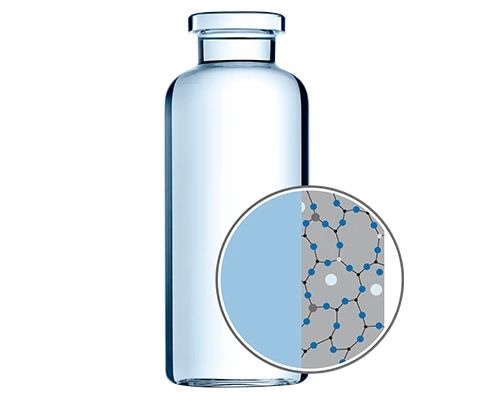
Drug containment solutions for rising biotech and biologics drug developers
Scientific progress is opening up completely new opportunities for biotech companies to treat and prevent diseases. For drug developers, this means having to master new analytical and technological challenges, but also to face new drug containment requirements to safely store highly sensitive biologics such as recombinant proteins or monoclonal antibodies.For smaller biotech companies, the right selection of drug containers is especially challenging since in-house capabilities are focused primarily on R&D and not on the selection of the optimal drug container. However, it is crucial to evaluate different primary packaging solutions early on in the drug development phase to avoid stability issues such as drug-container interaction in the commercialization phase.
Seven top challenges of drug-container interaction for biotechs
Challenge 1: Leachables
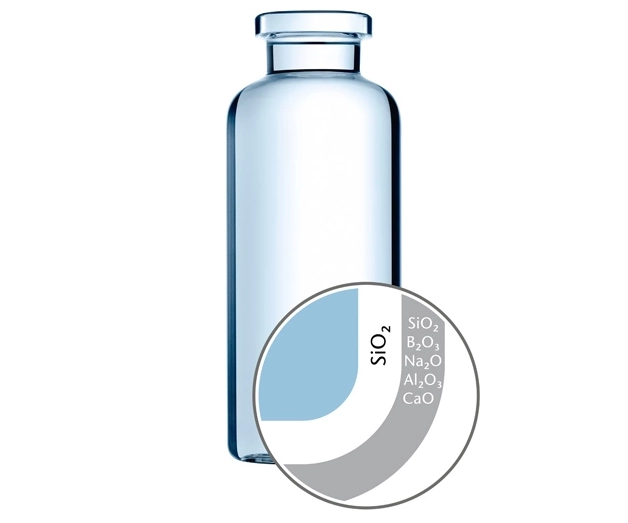
There is a potential exposure to leachable substances that could migrate from the pharmaceutical packaging or delivery system into the drug product, therefore it is important to assess the safety risk to the patient and any other potential issues posed by leachables. Leaching of glass elements poses a risk for highly sensitive biologics as leached-out ions might compromise the activity of the drug.
SCHOTT EVERIC® pure and SCHOTT Type I plus® glass vials offer a reduced leachable level. EVERIC® pure reduces leachables compared to a standard Type I container. SCHOTT Type I plus® reduces leachables even further due to the ion barrier. If you are interested, we also offer stability studies tailored to your needs.
Challenge 2: Protein adsorption
Proteins consist of different characteristics such as hydrophilic vs. hydrophobic or positively vs. negatively charged, which determine their tendency to adsorb to the primary packaging container. Big, complex proteins represent the largest share of biologics. The non-covalent adhesion of proteins to the glass surface can result in conformational changes, denaturation, aggregation, and loss of biological activity.
SCHOTT Type I plus® offers a specialized inner glass surface that can avoid protein adsorption and is also compliant with current standards.
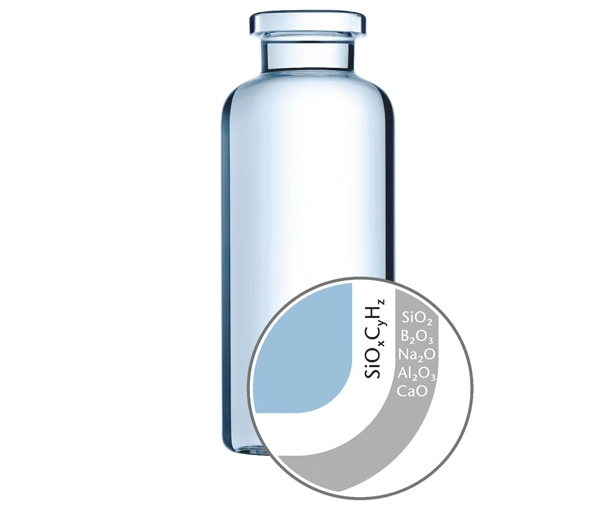
Challenge 3: Fogging
The majority of biologics will require lyophilization due to stability issues in liquid form. “Fogging” is a common observation of freeze-dried drug products. In addition to an unappealing appearance, severe fogging that reaches the vial’s shoulder or neck can potentially compromise container closure integrity. Antibody drug conjugates (ADCs) are especially known to exhibit fogging during lyophilization.
SCHOTT TopLyo® avoids fogging with a hydrophobic covalently bonded inner vial surface free of any residuals (e.g. silicone) known to initiate protein aggregation.

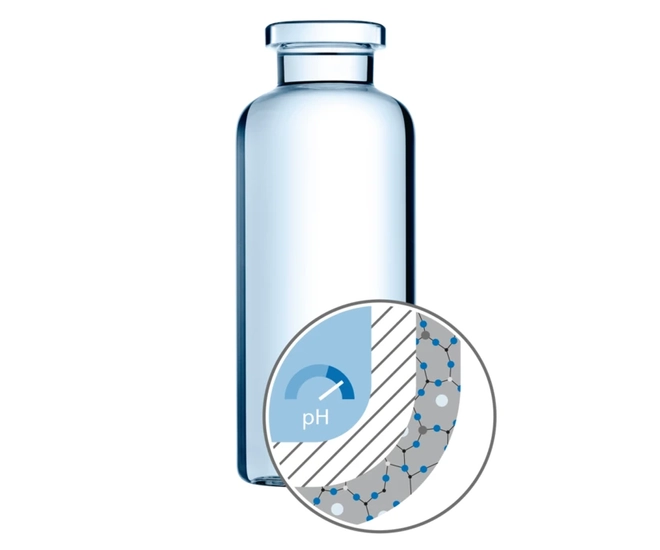
Challenge 4: pH shift
Changes of pH value can be the result of an exchange process between ions of the inner glass surface and protons of the liquid formulation. Consequences can be protein denaturation, as well as changes in the protein structure (folding or unfolding).
SCHOTT EVERIC® pure and Type I plus® glass vials are ideally suited to avoid pH shifts. EVERIC® pure reduces pH shift compared to a standard Type I container, while SCHOTT Type I plus® fully avoids pH shift due to the ion barrier present.
Challenge 5: Glass delamination
Glass delamination is the result of a local corrosion of the drug formulation with the heel zone of the vial. The most critical influencing factors for glass delamination are shelf life, temperature, special treatments as ammonium sulfate treatment and terminal sterilization, and the nature of the buffer.
EVERIC® pure is specifically highly recommended for phosphate buffers (aseptic processed) and terminally sterilized formulations such as diluents and water for injection (WFI) in general.
Challenge 6: Aluminum-sensitive drugs
Although aluminum is present in the human body, it is toxic if ingested in high amounts. Aluminum is a constituent of Type I glass and can therefore potentially leach into the drug product during its shelf life, posing a risk of introducing higher amounts of toxic aluminum into the body. Aluminum leaching is especially critical for parenteral nutrition containing amino acid.
The specialized inner glass surface of SCHOTT Type I plus® acts as an ion barrier, avoiding aluminum leaching.

Challenge 7: Radiopharmaceuticals
Radiopharmaceuticals consist of a chelator (complexing agent), a radionucleotide, and a tumor-targeting antibody. The chelator carries the radionucleotide but also has a strong affinity to any metal ion in solution. The ion barrier of SCHOTT Type I plus® vials ensures a stable complex between the radionucleotide and the chelator due to minimized ion leaching.
The specialized inner glass surface of SCHOTT Type I plus® acts as an ion barrier and avoids leaching of ions which will compete with the radionucleotide to form a complex with the chelator.
Selecting the right drug container for your high-value drugs
At SCHOTT, we produce a broad range of vials and syringes that are ideally suited for sensitive drugs such as complex proteins and vaccines. All of our products are manufactured and packed in environmentally controlled areas and conform to all major standards.
SCHOTT Vials
SCHOTT Syringes
syriQ BioPure®
The manufacturing process of syriQ BioPure® is designed to reduce the amount of tungsten and adhesive residues, as well as ensure a uniform silicone layer.
Save time using pre-washed and pre-sterilized vials
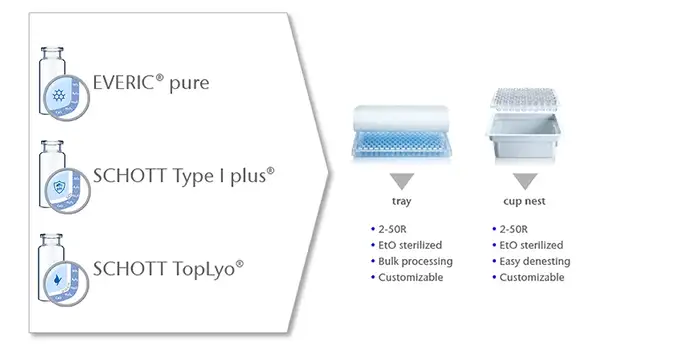
SCHOTT adaptiQ® is a ready-to-use vial solution that's compatible with over 50 different filling lines.
These days, no one should spend valuable time washing vials before using them. With adaptiQ®, we have specifically developed a ready-to-use solution for small to medium-sized biotech companies who do not have the facilities to wash and sterilize glass vials.
Made with SCHOTT FIOLAX® glass, adaptiQ® vials are washed and packaged in a clean room environment and sterilized with ethylene oxide. Compatible with over 50 types of filling lines, our brands EVERIC pure®, SCHOTT Type I plus®, and SCHOTT TopLyo® can all be shipped as ready-to-use options.
LEARN MORE ABOUT ADAPTIQ®
Download great insights on how to store highly sensitive drugs.
Learn all about the key considerations in selection a suitable drug containment solution.